Calorimeter Value Definition . calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. The process of measuring this heat is called. To do so, the heat is exchanged with a calibrated object. This requires careful measurement of. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change.
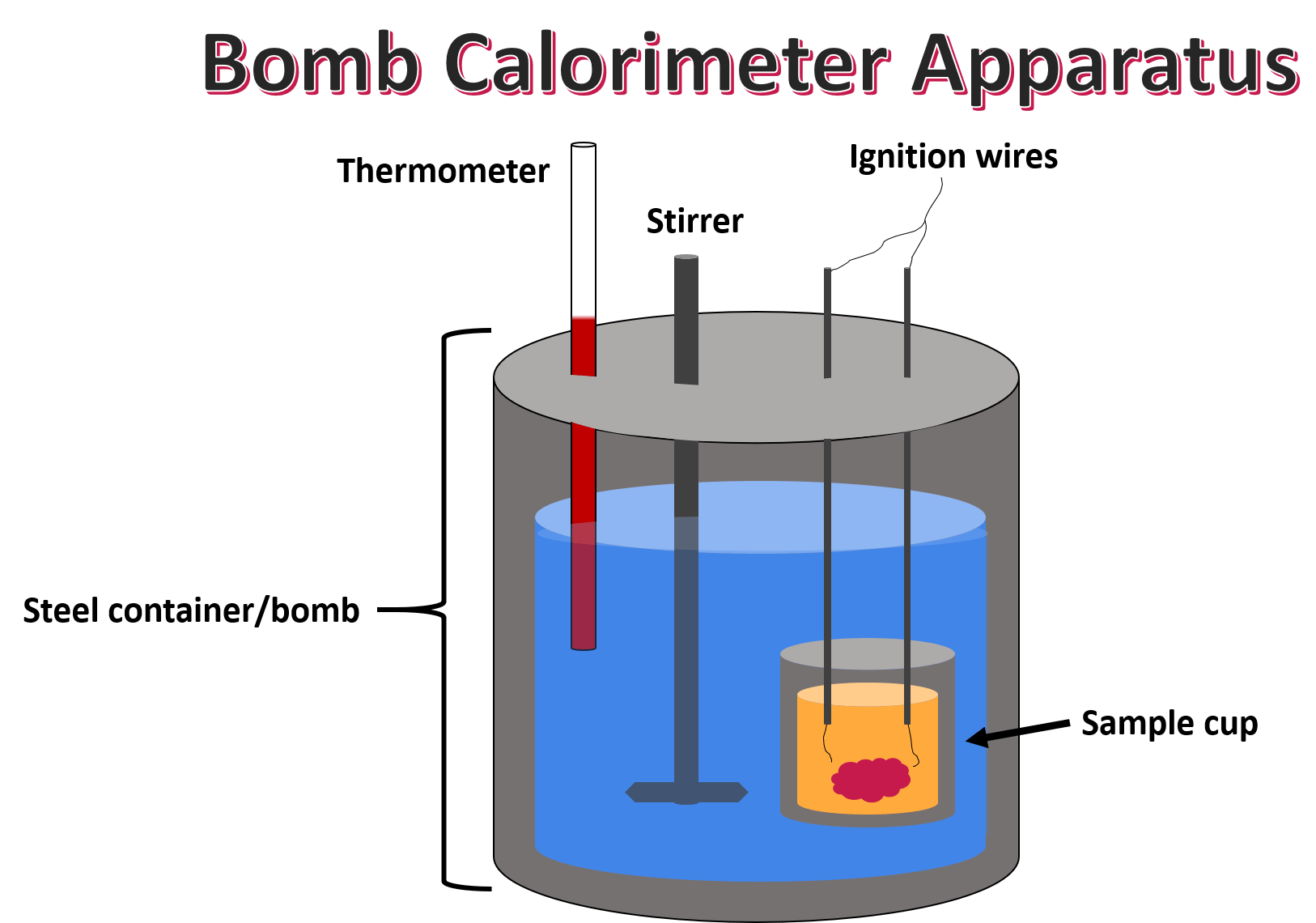
from cebkmocg.blob.core.windows.net
calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. To do so, the heat is exchanged with a calibrated object. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The process of measuring this heat is called. calorimetry is used to measure amounts of heat transferred to or from a substance. This requires careful measurement of. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
What Is A Bomb Calorimeter Definition at Donna Chaney blog
Calorimeter Value Definition calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. To do so, the heat is exchanged with a calibrated object. a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. This requires careful measurement of. The process of measuring this heat is called. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two.
From www.youtube.com
Principle of Calorimetry YouTube Calorimeter Value Definition To do so, the heat is exchanged with a calibrated object. The process of measuring this heat is called. This requires careful measurement of. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. calorimetry. Calorimeter Value Definition.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimeter Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a. Calorimeter Value Definition.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimeter Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. calorimetry is. Calorimeter Value Definition.
From studylib.net
Calorimetry Calorimeter Value Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. To do so, the heat is exchanged with a calibrated object. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between. Calorimeter Value Definition.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Value Definition calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calorimetry measures enthalpy changes during chemical. Calorimeter Value Definition.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimeter Value Definition The process of measuring this heat is called. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. Calorimeter Value Definition.
From www.chegg.com
Solved In the laboratory a "coffee cup" calorimeter, or Calorimeter Value Definition calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. To do so, the heat is exchanged. Calorimeter Value Definition.
From cescqsld.blob.core.windows.net
Heat Capacity Of Calorimeter Value at Jean Friend blog Calorimeter Value Definition calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. The. Calorimeter Value Definition.
From exodnulby.blob.core.windows.net
Lab Calorimetry And Specific Heat Summary at Barbara Bailey blog Calorimeter Value Definition calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. To do so, the heat is exchanged with a calibrated object. calorimetry is used to measure amounts of heat transferred to or from a substance.. Calorimeter Value Definition.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Calorimeter Value Definition This requires careful measurement of. The process of measuring this heat is called. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calculate heat, temperature change, and specific heat after. Calorimeter Value Definition.
From cezctooy.blob.core.windows.net
What Is Calorimetry Constant Pressure at Daryl Dirks blog Calorimeter Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. This requires careful measurement of.. Calorimeter Value Definition.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimeter Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. This requires careful measurement of. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. Calorimeter Value Definition.
From cebkmocg.blob.core.windows.net
What Is A Bomb Calorimeter Definition at Donna Chaney blog Calorimeter Value Definition calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. To do so, the heat is exchanged with a calibrated object. The. Calorimeter Value Definition.
From quizgrouchiest.z4.web.core.windows.net
Calorimeter Constant Definition Chemistry Calorimeter Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used to measure the heat flow of a chemical reaction or physical change. To do so, the heat is exchanged with a calibrated object. The process of measuring this heat is called. This requires. Calorimeter Value Definition.
From exoxbgvei.blob.core.windows.net
Calorimeter Laboratory at Clara Carter blog Calorimeter Value Definition calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. The process of measuring this heat is. Calorimeter Value Definition.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Value Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. The process of measuring this. Calorimeter Value Definition.
From cebkmocg.blob.core.windows.net
What Is A Bomb Calorimeter Definition at Donna Chaney blog Calorimeter Value Definition The process of measuring this heat is called. calculate heat, temperature change, and specific heat after thermal equilibrium is reached between two. To do so, the heat is exchanged with a calibrated object. calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is used to measure. Calorimeter Value Definition.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimeter Value Definition calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. calorimetry is used to measure the amount of thermal energy transferred in a chemical or physical process. calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change. a calorimeter is a device used. Calorimeter Value Definition.